Abstract
Introduction: Cutaneous T-cell lymphoma (CTCL) is an incurable malignancy of skin-homing T-cells with clinical manifestations that vary in severity and prognosis from isolated patch/plaque disease to tumors and systemic lymphoma with aggressive disease course. No therapy provides long-lasting responses and treatment options for advanced CTCL remain very limited. New targeted therapies are urgently needed. Monoclonal antibodies such as mogamulizumab (anti-CCR4), alemtuzumab (anti-CD52), nivolumab (anti-PD1), pembrolizumab (anti-PD1), and the drug-antibody conjugate brentuximab vedotin (anti-CD30) have provided significant advances in the treatment of advanced/refractory CTCL, though patients still ultimately progress. In this study, we investigate CD38, a biomarker that can be targeted with clinically approved antibodies. Additionally, we investigate the biological effects and anti-tumor immune mechanisms engaged by CD38 in CTCL pathogenesis.
Methods: First, we evaluated cell surface expression of CD38 in CTCL cell lines (N=4; HH, H9, Hut78, Hut102) and primary patient samples (N=7; from blood, cerebrospinal fluid, lymph nodes, and tonsils) using multiparameter flow cytometry. The seven CTCL samples included early stage mycosis fungoides (N=4) and aggressive leukemic variant Sézary Syndrome (N=3). To evaluate the therapeutic potential of targeting CD38 in CTCL in vivo, we transduced CD38+ CTCL cell line H9 with firefly luciferase-GFP lentivirus to allow for efficient purity sorting and in vivo monitoring using an in vivo imager system (IVIS). We engrafted H9 luciferase-expressing cells intravenously into immunodeficient NOD Rag-/-gc-/- (NRG) mice, treated them with the anti-CD38 antibody daratumumab or IgG control, and monitored disease progression over time with an IVIS. To study the effect of CD38 on CTCL growth, we used a CRISPR-Cas9 gene editing kit (Synthego) to generate either CD38 wild-type control (CD38WT) or CD38 knockout (CD38KO) H9 luciferase cells. Finally, a skin tumor model of CTCL was generated by engrafting either CD38WT or CD38KO H9 luciferase cells subcutaneously in NRG mice. Subcutaneous tumors were monitored for tumor growth using an IVIS imager. Flow cytometry analysis of tumors was performed to evaluate the immune microenvironment in CD38WT H9, and CD38KO H9 engrafted mice.
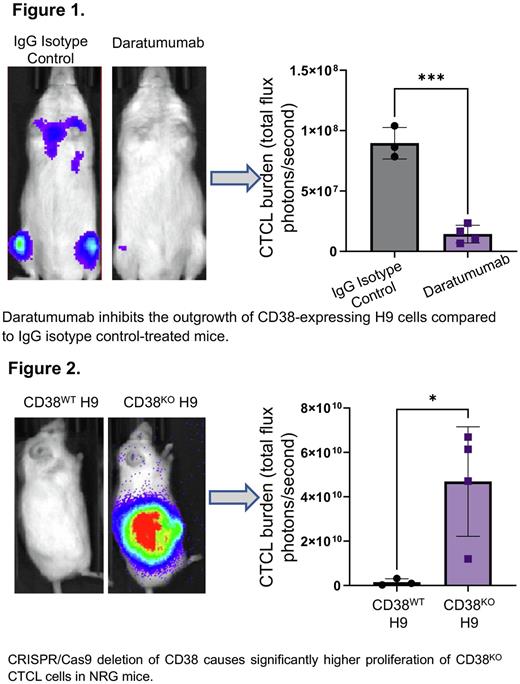
Results: Flow cytometry analysis demonstrated that patient-derived CTCL cell lines (HH, H9, Hut78, and Hut102) expressed high levels of CD38. Furthermore, all mycosis fungoides patient samples showed medium to high expression of CD38 on neoplastic cells suggesting the utility of CD38 as a therapeutic target for patients. Two out of three Sézary Syndrome patients demonstrated some CD38 expression, suggesting possible loss of this marker as the disease progresses. Pre-clinical testing of anti-CD38 antibody therapy against CD38wt H9 luciferase model mice showed that mice treated with daratumumab had significantly reduced malignant cell burden (as measured by total flux photons/second) compared to animals receiving IgG isotype control (Figure 1, p=0.0002). This demonstrates that daratumumab shows efficacy as a single-agent in a systemic model of CD38+ CTCL when administered after disease onset. Additionally, since our patient data shows lower CD38 expression in severe forms of CTCL, we generated a CD38KO H9 luciferase cell line to investigate the role of CD38 in CTCL pathogenesis. Our data showed that CD38KO H9 cells proliferated more aggressively than CD38WT H9 cells subcutaneously (Figure 2, p=0.0268). While it is currently unclear what role CD38 plays in CTCL pathogenesis to drive tumor progression, investigations into the immune microenvironment of CD38KO H9 tumors revealed an increased number of tumor infiltrating macrophages (CD11b+F4/80+) that were mostly of the immunosuppressive phenotype (CD206+) compared to CD38WT H9 tumors.
Conclusion: Overall, our studies demonstrate strong evidence for further investigation into the role of CD38 in the immunopathogenesis of CTCL and its value as a novel target for therapeutic intervention. Our data also provide preliminary evidence for the association between CD38 expression and disease progression in CTCL and suggest that the suppressive tumor microenvironment may contribute to disease progression.
Disclosures
Brammer:Bristol-Myers Squibb: Research Funding; Seattle Genetics: Speakers Bureau; Kymera Therapeutics: Consultancy; DrenBio: Consultancy. Porcu:Loxo: Membership on an entity's Board of Directors or advisory committees; ADCT: Membership on an entity's Board of Directors or advisory committees; Ono: Membership on an entity's Board of Directors or advisory committees; Teva: Honoraria, Research Funding; DrenBio: Consultancy; Morphosys: Membership on an entity's Board of Directors or advisory committees; BeiGene: Membership on an entity's Board of Directors or advisory committees; Innate Pharma: Honoraria, Membership on an entity's Board of Directors or advisory committees; Daiichi, Kyowa: Honoraria, Membership on an entity's Board of Directors or advisory committees; Viracta: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Nikbakht:Kyowa kirin: Membership on an entity's Board of Directors or advisory committees; Helsinn: Membership on an entity's Board of Directors or advisory committees, Research Funding. Mishra:Teva: Research Funding; Kymera: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal